ENZYMES
Almost all enzymes are proteins. There are some nucleic acids that behave like enzymes. These are called ribozymes. One can depict an enzyme by a line diagram. An enzyme like any protein has a primary structure, i.e., amino acid sequence of the protein. An enzyme like any protein has the secondary and the tertiary structure. The backbone of the protein chain folds upon itself, the chain criss-crosses itself and hence, many crevices or pockets are made. One such pocket is the ‘active site’.
An active site of an enzyme is a crevice or pocket into which the substrate fits. Thus enzymes, through their active site, catalyse reactions at a high rate. Enzyme catalysts differ from inorganic catalysts in many ways, but one major difference needs mention.
Inorganic catalysts work efficiently at high temperatures and high pressures, while enzymes get damaged at high temperatures (say above 40°C). However, enzymes isolated from organisms who normally live under extremely high temperatures (e.g., hot vents and sulphur springs), are stable and retain their catalytic power even at hightemperatures (upto 80°-90°C). Thermal stability is thus an important quality of such enzymes isolated from thermophilic organisms.
Chemical Reactions
How do we understand these enzymes? Let us first understand a chemical reaction. Chemical compounds undergo two types of changes. A physical change simply refers to a change in shape without breaking of bonds. This is a physical process. Another physical process is a change in state of matter: when ice melts into water, or when water becomes a vapour.
These are also physical processes. However, when bonds are broken and new bonds are formed during transformation, this will be called a chemical reaction. For example:
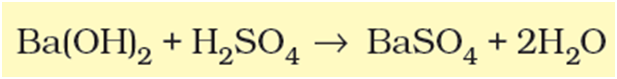
Catalysed reactions proceed at rates vastly higher than that of uncatalysed ones. When enzyme catalysed reactions are observed, the rate would be vastly higher than the same but uncatalysed reaction. For example

million times.
The power of enzymes is incredible indeed! There are thousands of types of enzymes each catalysing a unique chemical or metabolic reaction. A multistep chemical reaction, when each of the steps is catalysed by the same enzyme complex or different enzymes, is called a metabolic pathway. For example,
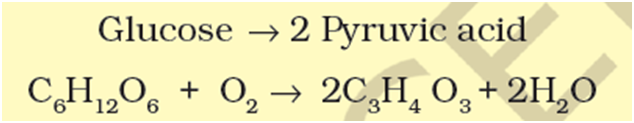
At this stage you should know that this very metabolic pathway with one or two additional reactions gives rise to a variety of metabolic end products. In our skeletal muscle, under anaerobic conditions, lactic acid is formed. Under normal aerobic conditions, pyruvic acid is formed. In yeast, during fermentation, the same pathway leads to the production of ethanol (alcohol). Hence, in different conditions different products are possible.
How do Enzymes bring about such High Rates of Chemical Conversions?
To understand this we should study enzymes a little more. We have already understood the idea of an ‘active site’. The chemical or metabolic conversion refers to a reaction. The chemical which is converted into a product is called a ‘substrate’. Hence enzymes, i.e. proteins with three dimensional structures including an ‘active site’, convert a substrate (S) into a product (P). Symbolically, this can be depicted as:

During the state where substrate is bound to the enzyme active site, a new structure of the substrate called transition state structure is formed. Very soon, after the expected bond breaking/making is completed, the product is released from the active site. In other words, the structure of substrate gets transformed into the structure of product(s).
The pathway of this transformation must go through the so-called transition state structure. There could be many more ‘altered structural states’ between the stable substrate and the product. Implicit in this statement is the fact that all other intermediate structural states are unstable. Stability is something related to energy status of the molecule or the structure.
The y-axis represents the potential energy content. The x-axis represents the progression of the structural transformation or states through the ‘transition state’. You would notice two things.
The energy level difference between S and P. If ‘P’ is at a lower level than ‘S’, the reaction is an exothermic reaction. One need not supply energy (by heating) in order to form the product. However, whether it is an exothermic or spontaneous reaction or an endothermic or energy requiring reaction, the ‘S’ has to go through a much higher energy state or transition state. The difference in average energy content of ‘S’ from that of this transition state is called ‘activation energy’. Enzymes eventually bring down this energy barrier making the transition of ‘S’ to ‘P’ more easy.
Nature of Enzyme Action
Each enzyme (E) has a substrate (S) binding site in its molecule so that a highly reactive enzyme-substrate complex (ES) is produced. This complex is short-lived and dissociates into its product(s) P and the unchanged enzyme with an intermediate formation of the enzyme-product complex (EP). The formation of the ES complex is essential for catalysis.

1. First, the substrate binds to the active site of the enzyme, fitting into the active site.
2. The binding of the substrate induces the enzyme to alter its shape, fitting more tightly around the substrate.
3. The active site of the enzyme, now in close proximity of the substrate breaks the chemical bonds of the substrate and the new enzyme- product complex is formed.
4. The enzyme releases the products of the reaction and the free enzyme is ready to bind to another molecule of the substrate and run through the catalytic cycle once again.
Factors Affecting Enzyme Activity
The activity of an enzyme can be affected by a change in the conditions which can alter the tertiary structure of the protein. These include temperature, pH, change in substrate concentration or binding of specific chemicals that regulate its activity.
Temperature and pH
Enzymes generally function in a narrow range of temperature and pH. Each enzyme shows its highest activity at a particular temperature and pH called the optimum temperature and optimum pH.
Activity declines both below and above the optimum value. Low temperature preserves the enzyme in a temporarily inactive state whereas high temperature destroys enzymatic activity because proteins are denatured by heat.
Concentration of Substrate
With the increase in substrate concentration, the velocity of the enzymatic reaction rises at first. The reaction ultimately reaches a maximum velocity (Vmax) which is not exceeded by any further rise in concentration of the substrate. This is because the enzyme molecules are fewer than the substrate molecules and after saturation of these molecules, there are no free enzyme molecules to bind with the additional substrate molecules .
The activity of an enzyme is also sensitive to the presence of specific chemicals that bind to the enzyme. When the binding of the chemical shuts off enzyme activity, the process is called inhibition and the chemical is called an inhibitor.
When the inhibitor closely resembles the substrate in its molecular structure and inhibits the activity of the enzyme, it is known as competitive inhibitor. Due to its close structural similarity with the substrate, the inhibitor competes with the substrate for the substrate binding site of the enzyme.
Consequently, the substrate cannot bind and as a result, the enzyme action declines, e.g., inhibition of succinic dehydrogenase by malonate which closely resembles the substrate succinate in structure. Such competitive inhibitors are often used in the control of bacterial pathogens.
Classification and Nomenclature of Enzymes
Thousands of enzymes have been discovered, isolated and studied. Most of these enzymes have been classified into different groups based on the type of reactions they catalyse. Enzymes are divided into 6 classes each with 4-13 subclasses and named accordingly by a four-digit number.
Oxidoreductases/dehydrogenases: Enzymes which catalyse oxidoreduction between two substrates S and S’ e.g.,


Lyases: Enzymes that catalyse removal of groups from substrates by mechanisms other than hydrolysis leaving double bonds.

Ligases: Enzymes catalysing the linking together of 2 compounds, e.g., enzymes which catalyse joining of C-O, C-S, C-N, P-O etc. bonds.
Co-factors
Enzymes are composed of one or several polypeptide chains. However, there are a number of cases in which non-protein constituents called cofactors are bound to the the enzyme to make the enzyme catalytically active. In these instances, the protein portion of the enzymes is called the apoenzyme.
Three kinds of cofactors may be identified: prosthetic groups, co-enzymes and metal ions. Prosthetic groups are organic compounds and are distinguished from other cofactors in that they are tightly bound to the apoenzyme. For example, in peroxidase and catalase, which catalyze the breakdown of hydrogen peroxide to water and oxygen, haem is the prosthetic group and it is a part of the active site of the enzyme.
Co-enzymes are also organic compounds but their association with the apoenzyme is only transient, usually occurring during the course of catalysis. Furthermore, co-enzymes serve as co-factors in a number of different enzyme catalyzed reactions. The essential chemical components of many coenzymes are vitamins, e.g., coenzyme nicotinamide adenine dinucleotide (NAD) and NADP contain the vitamin niacin.
A number of enzymes require metal ions for their activity which form coordination bonds with side chains at the active site and at the same time form one or more cordination bonds with the substrate, e.g., zinc is a cofactor for the proteolytic enzyme carboxypeptidase.
Catalytic activity is lost when the co-factor is removed from the enzyme which testifies that they play a crucial role in the catalytic activity of the enzyme.
