BIOMOLECULES – HOW TO ANALYSE CHEMICAL COMPOSITION?
We can continue asking in the same way, what type of organic compounds are found in living organisms? How does one go about finding the answer? To get an answer, one has to perform a chemical analysis. We can take any living tissue (a vegetable or a piece of liver, etc.) and grind it in trichloroacetic acid (Cl3CCOOH) using a mortar and a pestle. We obtain a thick slurry. If we were to strain this through a cheesecloth or cotton we would obtain two fractions.
One is called the filtrate or more technically, the acid-soluble pool, and the second, the retentate or the acid-insoluble fraction. Scientists have found thousands of organic compounds in the acid-soluble pool.
In higher classes you will learn about how to analyse a living tissue sample and identify a particular organic compound. It will suffice to say here that one extracts the compounds, then subjects the extract to various separation techniques till one has separated a compound from all other compounds. In other words, one isolates and purifies a compound.
Analytical techniques, when applied to the compound give us an idea of the molecular formula and the probable structure of the compound. All the carbon compounds that we get from living tissues can be called ‘biomolecules’. However, living organisms have also got inorganic elements and compounds in them. How do we know this? A slightly different but destructive experiment has to be done. One weighs a small amount of a living tissue (say a leaf or liver and this is called wet weight) and dry it. All the water, evaporates.
The remaining material gives dry weight. Now if the tissue is fully burnt, all the carbon compounds are oxidised to gaseous form (CO2, water vapour) and are removed. What is remaining is called ‘ash’. This ash contains inorganic elements (like calcium, magnesium etc). Inorganic compounds like sulphate, phosphate, etc., are also seen in the acid-soluble fraction.
Therefore elemental analysis gives elemental composition of living tissues in the form of hydrogen, oxygen, chlorine, carbon etc. while analysis for compounds gives an idea of the kind of organic (Figure 9.1) and inorganic constituents (Table 9.2) present in living tissues. From a chemistry point of view, one can identify functional groups like aldehydes, ketones, aromatic compounds, etc. But from a biological point of view, we shall classify them into amino acids, nucleotide bases, fatty acids etc. Amino acids are organic compounds containing an amino group and an acidic group as substituents on the same carbon i.e., the a-carbon.
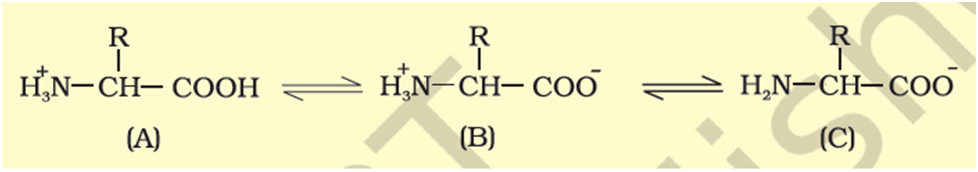
Hence, they are called a-amino acids. They are substituted methanes. There are four substituent groups occupying the four valency positions. These are hydrogen, carboxyl group, amino group and a variable group designated as R group. Based on the nature of R group there are many amino acids. However, those which occur in proteins are only of twentytypes. The R group in these proteinaceous amino acids could be a hydrogen (the amino acid is called glycine), a methyl group (alanine), hydroxy methyl (serine), etc.The chemical and physical properties of amino acids are essentially of the amino, carboxyl and the R functional groups. Based on number of amino and carboxyl groups, there are acidic (e.g., glutamic acid), basic (lysine) and neutral (valine) amino acids. Similarly, there are aromatic amino acids (tyrosine, phenylalanine, tryptophan). A particular property of amino acids is the ionizable nature of –NH2 and –COOH groups. Hence in solutions of different pH, the structure of amino acids changes.
Fatty acids could be saturated (without double bond) or unsaturated (with one or more C=C double bonds). Another simple lipid is glycerol which is trihydroxy propane. Many lipids have both glycerol and fatty acids. Here the fatty acids are found esterified with glycerol. They can be then monoglycerides, diglycerides and triglycerides. These are also called fats and oils based on melting point. Oils have lower melting point (e.g., gingelly oil) and hence remain as oil in winters. Can you identify a fat from the market? Some lipids have phosphorous and a phosphorylated organic compound in them. These are phospholipids. They are found in cell membrane. Lecithin is one example. Some tissues especially the neural tissues have lipids with more complex structures.
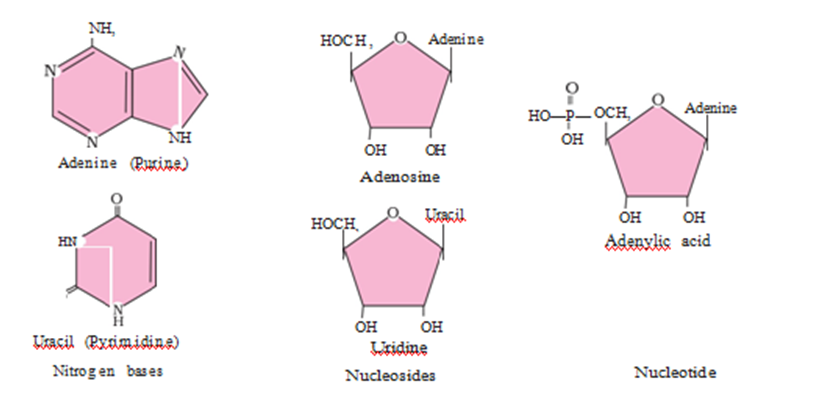
Living organisms have a number of carbon compounds in which heterocyclic rings can be found. Some of these are nitrogen bases – adenine, guanine, cytosine, uracil, and thymine. When found attached to a sugar, they are called nucleosides. If a phosphate group is also found esterified to the sugar they are called nucleotides. Adenosine, guanosine, thymidine, uridine and cytidine are nucleosides. Adenylic acid, thymidylic
acid, guanylic acid, uridylic acid and cytidylic acid are nucleotides. Nucleic acids like DNA and RNA consist of nucleotides only. DNA and RNA function as genetic material.